News
In-House Engineering Team in Pharma Cold Stores
At CRS Pharma Solutions, we are driven by a deep sense of pride in our ability to deliver innovative portable cold stores and blast freezers to meet the demands of our esteemed customers. What sets us apart is our unique offering of...
Streamlining Logistics: Cross-Docking Cold Store
In the world of supply chain management, efficiency and speed are crucial for businesses to stay competitive. When it comes to industries that rely on temperature-controlled storage, such as retail, logistics, pharmaceuticals, and the food and...
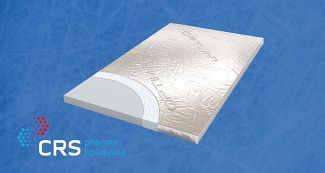
Vacuum Insulated Panels (VIPs)
Vacuum Insulated Panels (VIPs) are specially designed panels that have the thermal insulation of a vacuum in a board form. As the vacuum has no thermal conductivity, these are considered the next generation of insulation products! With such...
In-house Stability Storage
Justification for bringing back in-house Stability Storage trials Stability storage is where samples or finished products are placed in specific environmental conditions, albeit temperature and humidity or cold and frozen climate. This is...
Concerns over insufficient Medicine Cold Storage
Maintaining precise temperatures for various medicines can be challenging when employing incorrect storage. A high-quality cold storage solution is required to secure the quality and effectiveness of various medicines. CRS offer a wide range of...
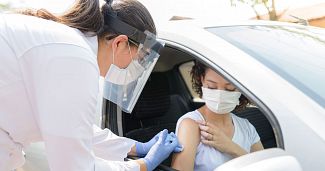
COVID Booster Handling and Distribution
With the Covid-19 booster becoming available to the masses, distributors need to consider how It can be handled, distributed, and stored long-term in line with the rising demand. At CRS, our experience within the pharmaceutical industry enabled us...
The Benefits of Pharma Cold Storage
The pharmaceutical industry heavily relies on ensuring that medicine, chemicals and other substances remain viable for their intended purpose. For many of these products, temperature plays a vital role in keeping their efficacy and suitability...
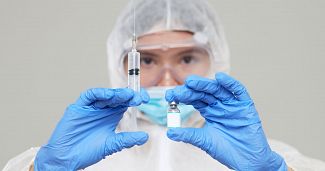
The Global COVID Vaccine Distribution
With the COVID-19 outbreak officially declared a pandemic on the 11th March 2020, the virus has spread worldwide with some significant efforts in both slowing down COVID-19 and finding a viable vaccine. The UK and US are taking a leading position...
The Demand for -70 Ultra Low Storage
Delivering highly specified temperature-controlled storage solutions to the pharmaceutical industry for several years, we have developed a strong experience in producing solutions to meet the stringent requirements of products. Demand for...
-70/80° ultra-low temperature storage
If you have read our recent article on The COVID-19 Vaccine, you will understand the complexity of storing a vaccine. Once it has been developed, precise conditions are required for storage to retain its efficacy. One of the main factors...
Page 1 of 4